7 Hours of networking time
30+ presentations from industry and academic leaders
Poster Presentations and Flash Talk Sessions
7 Hours of networking time
30+ presentations from industry and academic leaders
Poster Presentations and Flash Talk Sessions
Explore how a high fat diet leads to pathological interactions between microbes generate toxic metabolites that potentially divert TGF-β signaling, triggering HCC
Understand how knowledge of epigenetic and epitranscriptomics modifiers can help individual risk stratification and provide the basis for prevention and treatment strategies
Understand how the use of neoepitope markers of the extracellular matrix could improve patient endotyping & help evaluate therapeutical effects in MASLD






































| Date | Track 1 | Track 2 | ||
|---|---|---|---|---|
|
Day 1- 24th June
|
Novel Biomarkers and Diagnostics
|
Clinical Development and Clinical Trial Data
|
||
|
Day 2- 25th June
|
Recent updates in MASH pathogenesis
|
Clinical Development and Clinical Trial Data
|








Global Engage is committed to hosting sustainable meetings.
We have reduced waste by replacing printed documentation with an app. Make sure you have it downloaded to your devices in time for the meeting.
You will have some great food choices while you are with us but now, we have worked with the caterer to increase the proportion of plant-based items. We have also built a plan with the venue to avoid waste through how they serve meals and how leftovers are processed.
An international meeting does involve travel but where it is practical, please consider more sustainable alternatives to flying. The app will also have a discussion space to arrange ride shares.
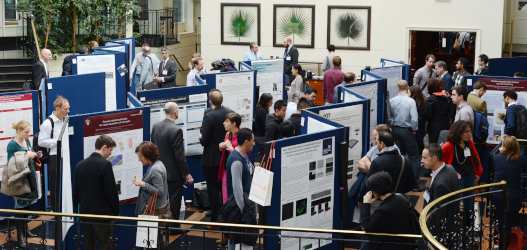
Whether looking for funding, job opportunities or simply wanting to share your work with a like-minded and focused group, poster presentations are an excellent way to join the heart of the congress.
Your presentation will be displayed in a dedicated area, with other posters from industry and academia.
New for 2024- We have reserved 2 x 50-minute sessions for non-vendor authors to present a flash oral presentation of their poster in order to showcase their work.
POSTER SUBMISSION FORM– CLOSING DATE: 7th June 2024
Sponsor a Global Engage meeting to access senior professionals in the life science industry and build your brand. Our events offer extensive networking opportunities, workshops, speaking positions, one-to-one delegate meetings, and exhibition booths to showcase your products and services. Gain a competitive advantage and position your company as a thought leader. Call us at UK +44 (0)1865 849841 Malaysia +603 2779 0098 to learn more.
Only products with same currency can be added to the basket. Clear the basket or finish the order, before adding products with another currency to the basket.