Presentation given by Scott Harris, the Chief Medical Officer of Altimmune
Scott Harris, the Chief Medical Officer of Altimmune, gave a keynote address at the 6th Global NASH Congress where he discussed the development of drugs for both obesity and the treatment of NASH. He began by describing the statistics on obesity in the US and the lack of effective and safe therapies for the treatment of obesity in the past. He then highlighted the recent breakthroughs in incretin-based therapies, such as GLP-1, GLP-1/GIP, and GLP-1/glucagon dual agonists, which have shown promising results in clinical trials.
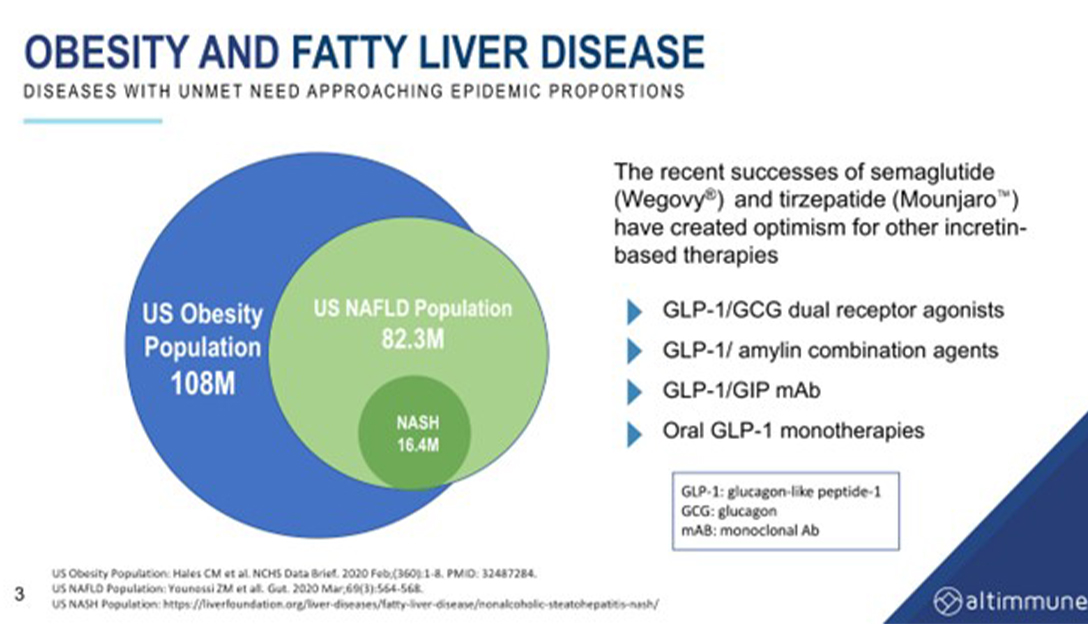
He noted that up until recently, catecholamine-based agents were predominantly used for weight loss, but they were associated with safety concerns and were not effective. Incretin-based therapies, on the other hand, have been shown to achieve significant weight loss in clinical trials. GLP-1, for example. By starting with the GLP-1 backbone and adding agents to drive greater weight loss, researchers are developing new therapies for obesity and NASH.
One such drug in development is tirzepatide, which combines GLP-1 and GIP to drive greater weight loss. Scott also mentioned oral semaglutide (Rybelsus), which is a small molecule GLP-1 that can be given orally very effectively, although the weight loss achieved is not as great as other agents.
The presentation emphasized the importance of clinical trials in both pure obesity and NASH to understand the optimal treatments for these conditions. He highlighted the various clinical trials that have shown the effectiveness of incretin-based therapies in achieving weight loss and reducing liver fat. He also noted that obesity and NASH are closely related, and that treating obesity can have beneficial effects on NASH. However, weight loss alone may not be sufficient to treat NASH, and researchers are still exploring the optimal treatments for this condition.
Overall, Scott provided technical details on the different classes of drugs used to treat obesity and NASH, as well as some of the specific drugs currently in development. He also discussed the importance of clinical trials in advancing our understanding of these conditions and developing effective treatments.
The presentation by Rohit Loomba in 2020 discussed the relationship between the reduction of liver fat induced by a drug and the improvement of NASH parameters. An ROC analysis was performed, which suggested that a reduction of liver fat of at least 41.5% is required to achieve NASH resolution and fibrosis improvement. However, achieving this reduction quickly is equally as important, particularly in trials with an endpoint of a biopsy at one year, as the liver needs to heal quickly.
Resmetirom, an FXR agonist, was found to induce a reduction in liver fat of approximately 50% in clinical trials. In Phase 3 trials, it achieved statistical significance for both NASH resolution and fibrosis improvement. However, many compounds have not exceeded the 41.5% reduction threshold, with the FXRs typically achieving reductions in the range of 35%.
Newer generation compounds such as FGF 21s are achieving reductions in liver fat content of 60-70%, which is above the original compounds that were developed to treat NASH.
Semaglutide is a GLP-1 monotherapy that was studied in a trial for the treatment of NASH. The highest arm of the trial involved a weekly dose of 2.4 milligrams of Wegovy, a GLP-1 agonist. Approximately 50-60% of the patients in the trial had diabetes, which can affect weight loss outcomes in obesity trials. The trial showed a mean weight loss of about 12.5%, which is lower than the 15-16% achieved in pure non-diabetes trials with Wegovy.
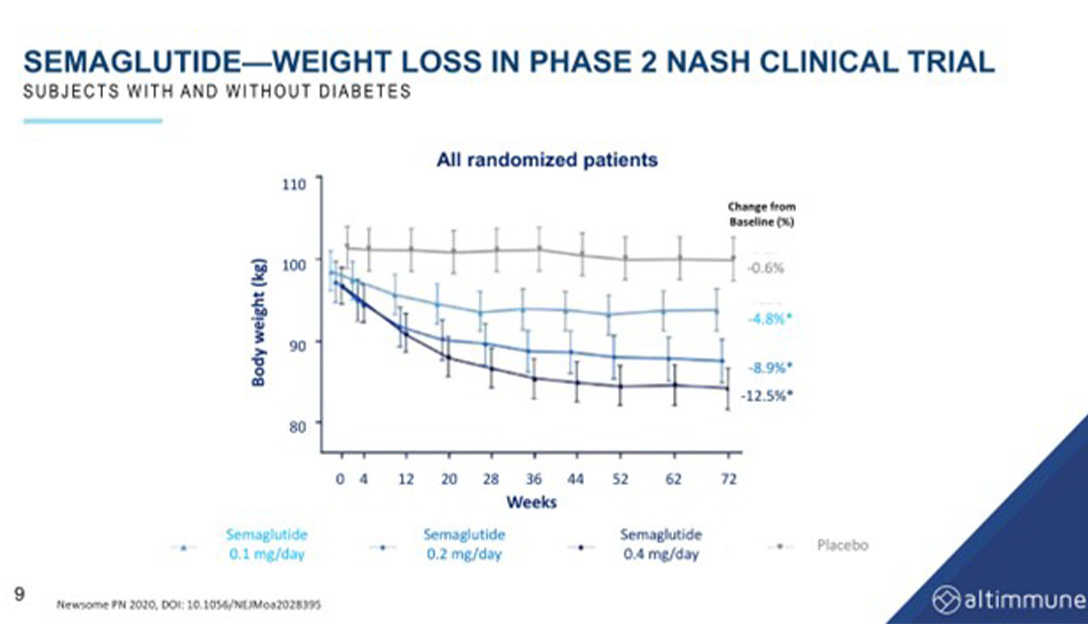
While semaglutide was found to be effective in achieving NASH resolution, it did not improve fibrosis. The reduction of liver fat induced by semaglutide is slow, with a reduction of approximately 34% at 24 weeks and 57% at the end of a year. This reduction is entirely due to weight loss, as there are no GLP-1 receptors in the liver. The same is true for tirzepatide and GIP, which also induce liver fat reduction solely through weight loss.
Fibrosis improvement is slow, taking as long as five years to achieve significant improvement. If a sufficient reduction of liver fat is not achieved in a short period of time, it is unlikely to have an effect on fibrosis in a one-year trial.
GLP-1 agonists such as semaglutide and GIP work by reducing appetite, while glucagon increases resting energy expenditure. The combination of GLP-1 and glucagon may be beneficial in the treatment of NASH, as there are glucagon receptors in the liver, which offers the potential for potent reduction of liver fat. Glucagon may also increase adipose tissue browning and other factors that can be synergistic to reducing calories alone.
The presentation discussed the potential of GLP-1/ glucagon dual receptor agonists for the treatment of NASH, a disease that is becoming increasingly common due to the rise of obesity and diabetes.
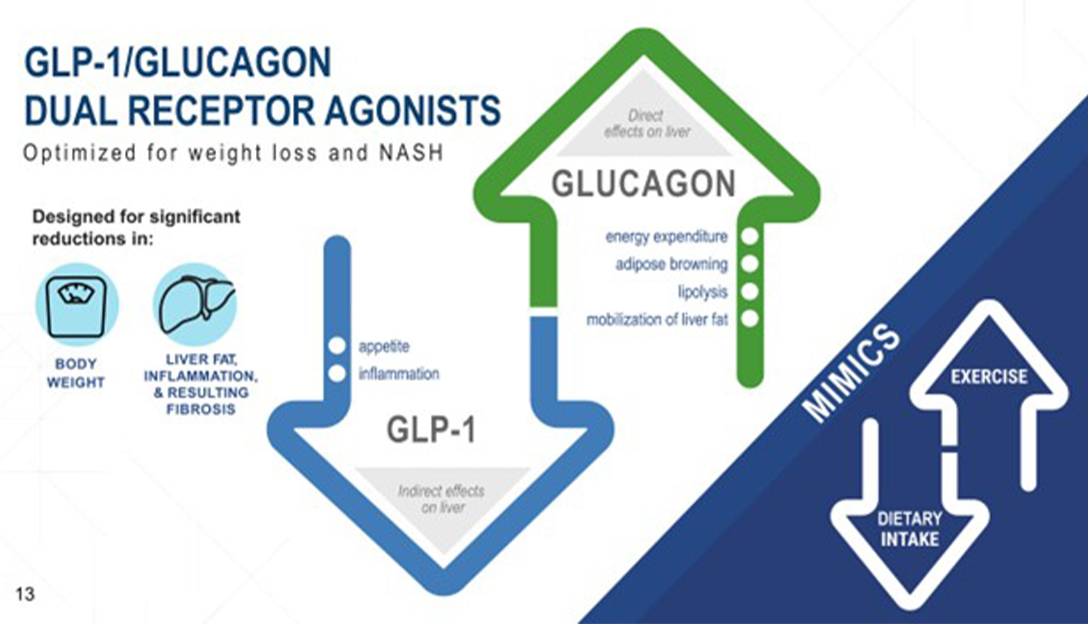
Scott explained that glucagon, a hormone produced by the pancreas, can reduce lipid synthesis in the liver and increase lipid mobilization and lipolysis. He suggests that combining glucagon with a GLP-1 backbone could be a highly effective treatment for NASH. Harris then presents data on pemvidutide, a GLP-1/ glucagon dual receptor agonist, which has been shown to reduce liver fat by 75% within 24 weeks of treatment. This reduction in liver fat may be able to achieve good biopsy results in a shorter period of time on fibrosis.
He continued to explain that GLP-1/ glucagon dual receptor agonists work by combining two compounds, GLP-1 and glucagon, in the same molecule. The ratio of GLP-1 to glucagon in the molecule determines how much glucagon activity it has. Pemvidutide distinguishes itself from other GLP-1/ glucagon dual receptor agonists by having a one-to-one ratio, which means it has the highest glucagon activity of any of the GLP-1 glucagon dual receptor agonists that are currently available.
In his presentation, Scott noted the importance of pharmacokinetics in these types of drugs. The faster a molecule gets into the bloodstream from a subcutaneous injection, the more side effects it may have. Pemvidutide has a side chain that slows its entry into the bloodstream, which may allow for better tolerability and fewer side effects.
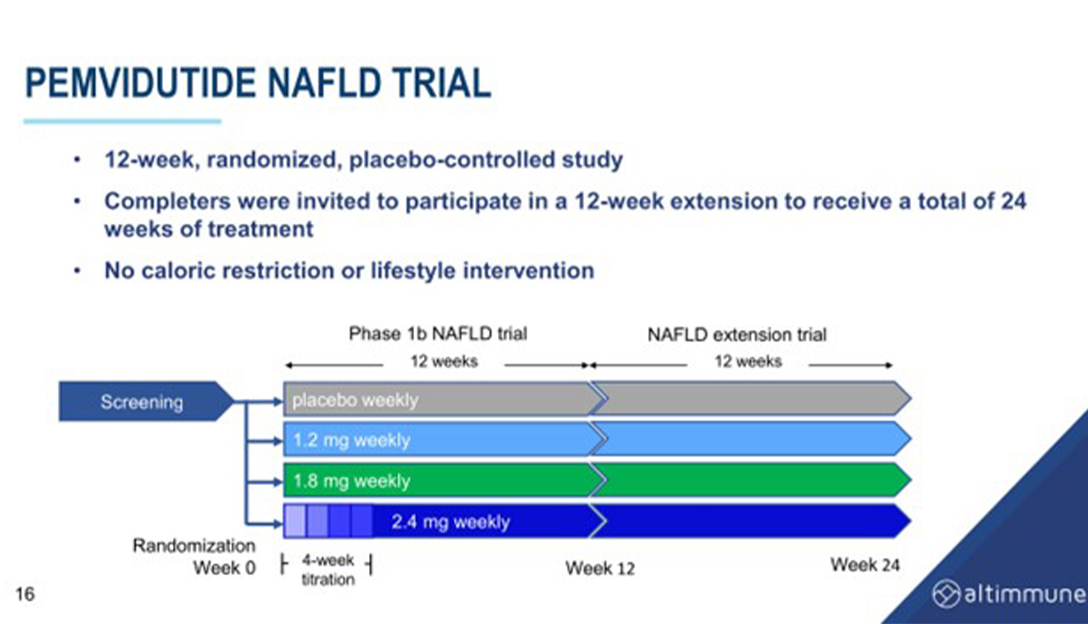
Data from a 12-week trial of pemvidutide in NAFLD showed that pemvidutide reduced liver fat content by 70% within 24 weeks and achieved high rates of 30% and 50% reductions, with over 50% of people achieving normalization of liver fat. Ppemvidutide also showed significant reductions in ALT and cCT1, markers of liver inflammation, and was associated with a reduction in serum lipids and blood pressure with minimal changes on heart rate.
Scott compared pemvidutide to other GLP-1-based drugs in development for NASH and obesity, including semaglutide, tirzepatide, cotadutide, and BI 456906. He notes that pemvidutide has the highest ratio of glucagon to GLP-1, making it highly effective for reducing liver fat, but may not be as effective for weight loss as some of the other drugs. He also stressed the importance of weight loss, reduction of liver fat and lipids, reduced liver inflammation, blood pressure, and tolerability in the treatment of NASH.
In summary, Harris’s speech focuses on the potential of GLP-1/glucagon dual receptor agonists for the treatment of NASH, a type of liver disease that is becoming increasingly common due to the rise of obesity and diabetes. He presented data on pemvidutide, a GLP-1/glucagon dual receptor agonist with a one-to-one ratio of GLP-1 to glucagon, which has been shown to reduce liver fat by 75% within 24 weeks of treatment. Harris also emphasized the importance of pharmacokinetics and tolerability in these types of drugs and presents data from a 12-week trial.
This article was written by Nick Noakes summarising the presentation given by Scott Harris, the Chief Medical Officer of Altimmune.





